Abbkine Scientific unravels an exclusive Ni-Super Resin portfolio under the Purification tools category called PurKine™ His-Tag Ni-Super Resin which is said to be featured two prominent advantages compared to Ni-NTA and Ni-IDA, high tolerance and easy cleaning.
Expression and purification of recombinant protein is the foundation of studying protein structure, regulation and function. To simplify purification, affinity purification tags are often fused to a recombinant protein of interest.The most popular tag is the polyhistidine (6xHis) tag. Some proteins, like eukaryotic proteins may not exhibit proper protein activity or folding if expressed in E.coli . Cultured mammalian cells or insect cells might be better option. However, the low expression levels of recombinant proteins in those cells presents a challenge for their purification.

Structure of Ni-IDA, Ni-NTA and Ni-Super shows Ni-Super has two prominent advantages compared to Ni-NTA and Ni-IDA with higher tolerance and easier cleaning.
Abbkine PurKine™ Ni-Super Resin is designed mainly for capturing and purification of histidine-tagged proteins secreted into eukaryotic cell, such as yeast, mammalian, and insect culture supernatants. PurKine™ Ni-Super Resin is a new immobilized metal ion affinity chromatography (IMAC) medium precharged with nickel ions via a proprietary, chemical-stable linker technology. The Resin consists of 90um beads of highly cross-linked 4% agarose, to which Super replacement to NTA has been coupled. Compared to Ni-NTA and Ni-IDA, Ni-Super resin has prominent advantages.
- High toleration— More tolerable to various chemicals including reducing, chelating agents. The nickel ions have been shown to remain bound to the chelating ligand even after incubation for 24 hours in 10 mM EDTA.
- Low nickel ion leaching— Best option for samples that can cause Ni stripping from the medium, like secreted proteins in liquids containing chelators. Do not need repeated regeneration.
- Time-saving sample preparation— Direct loading of samples without extra pre-treatment procedures, enabling purification of low concentrations of target proteins at large volumes.
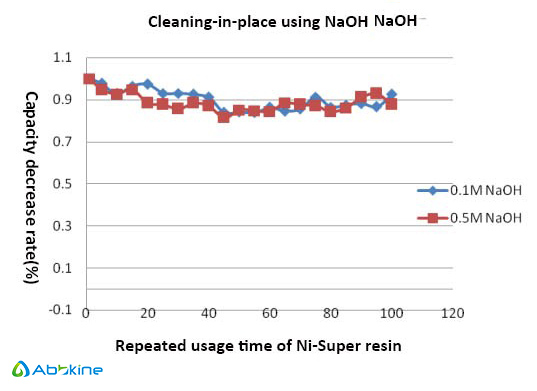
- Easy cleaning— Directly use NaOH to cleaning-in-place, reducing cleaning cycle.
Ni-Super resin is more tolerable to various chemicals including reducing, chelating agents. The nickel ions have been shown to remain bound to the chelating ligand even after incubation for 24 hours with 10 mM EDTA. When cleaning, directly use NaOH. It is easy to pack and use, and its fast flow properties make it excellent for scaling-up.

PurKine™ His-Tag Purification system is based on innovative high-capacity IMAC matrix for convenient single-step purifications of His-tag proteins from total lysates. Our propriety ion-chelate chemistry ensures extraordinary compatibility with commonly used reducing agents such as DTT, chelating metalloprotease inhibitors such as EDTA, and a wide range of buffer substances and salt conditions. The portfolio provides wide choice of chelating group (IDA, NTA or Super NTA), cross-linked agarose (crosslinked 4% or 6% agarose), ions (Nick, Colbat, Copper, or non ion charged) and compatible ingredients to allow optimization of process for maximum protein yield, stability and solubility. Tests also confirm that no decrease in performance occurs after at least five repeated uses. The PurKine™ His-Tag Purification resin is also available as prepacked spin column and kit formats.
Abbkine Scientific Co, Ltd was founded by a number of scientists and marketing experts in the field of life science in California in 2012. With growing demands from Asia Pacific, it move its headquarters to China. Abbkine started featured and exclusive products from protein detection tools, and devoted to developing and providing the most innovative solutions for global customers covering all kinds of antibodies, biochemicals, proteins and assay kits.
 binds to streptavidin with a dissociation constant of 4 nM and the Nano-tag(9) is a 9-mer peptide with a dissociation constant of 17 nM.
binds to streptavidin with a dissociation constant of 4 nM and the Nano-tag(9) is a 9-mer peptide with a dissociation constant of 17 nM. If you’re working in the life sciences with VSV-G tag in your vector or fusion proteins, chances are you’re going to be using right VSV-G antibodies in your research. Here we’ve set out some top tips to get you started in making a good and suitable VSV-G tag antibody buying decision.
If you’re working in the life sciences with VSV-G tag in your vector or fusion proteins, chances are you’re going to be using right VSV-G antibodies in your research. Here we’ve set out some top tips to get you started in making a good and suitable VSV-G tag antibody buying decision.

 fluorescent proteins (FPs) isolated from coral reef organisms; green fluorescent protein (GFP), derived from the jellyfish Aequorea victoria, is its most famous representative.AmCyan has been
fluorescent proteins (FPs) isolated from coral reef organisms; green fluorescent protein (GFP), derived from the jellyfish Aequorea victoria, is its most famous representative.AmCyan has been
 sensors of which the most likely donor FP is mCFP (monomeric cyan FP).
sensors of which the most likely donor FP is mCFP (monomeric cyan FP).
 of recombinant proteins. Anti-Strep-tag II antibodies are ideal for investigators involved in GFP and Epitopes research.
of recombinant proteins. Anti-Strep-tag II antibodies are ideal for investigators involved in GFP and Epitopes research.
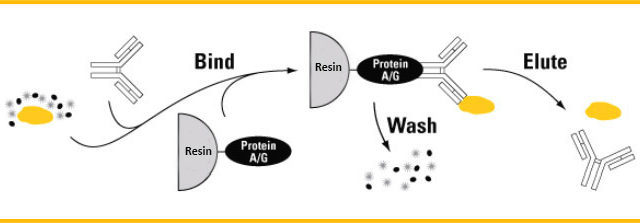
 Protein At is an alkali-resistant protein A (Alkaline tolerate) based on the native protein A though biotechnology mutation. Protein At Resin has been immobilized for the capture of monoclonal antibodies at process scale. Protein At Resin provides greater stability than conventional protein A-based resin in alkaline conditions used in cleaning-in-place (CIP) . The enhanced alkali stability of protein At Resin improves process economy and product quality.
Protein At is an alkali-resistant protein A (Alkaline tolerate) based on the native protein A though biotechnology mutation. Protein At Resin has been immobilized for the capture of monoclonal antibodies at process scale. Protein At Resin provides greater stability than conventional protein A-based resin in alkaline conditions used in cleaning-in-place (CIP) . The enhanced alkali stability of protein At Resin improves process economy and product quality. method including the combination of FLAG tags and HA tags.The TAP method permits the identification of proteins interacting with a particular target protein without any prior knowledge about the function, activity, or composition of the interacting proteins. This tag is also known as the C-terminal TAP tag because an N-terminal version is also available. However, the method to be described assumes the use of a C-terminal tag, although the principle behind the method is still the same.
method including the combination of FLAG tags and HA tags.The TAP method permits the identification of proteins interacting with a particular target protein without any prior knowledge about the function, activity, or composition of the interacting proteins. This tag is also known as the C-terminal TAP tag because an N-terminal version is also available. However, the method to be described assumes the use of a C-terminal tag, although the principle behind the method is still the same.





 extracts through CaM affinity resin. CBP Tag antibody is a useful tool in analysis of CBP-tagged proteins.
extracts through CaM affinity resin. CBP Tag antibody is a useful tool in analysis of CBP-tagged proteins.


 trans Golgi bodies without being transported between them in vesicles, supporting the cisternal maturation model of Golgi trafficking.
trans Golgi bodies without being transported between them in vesicles, supporting the cisternal maturation model of Golgi trafficking.
 demonstrated that the V5 epitope was displayed on the surface ofthe capsid protein. Furthermore, the recombinant marker virus behaved similarly to the parental virus in vitro and in mice, and could be differentiated from the parental virus via polymerase chain reaction (PCR) and serological methods.
demonstrated that the V5 epitope was displayed on the surface ofthe capsid protein. Furthermore, the recombinant marker virus behaved similarly to the parental virus in vitro and in mice, and could be differentiated from the parental virus via polymerase chain reaction (PCR) and serological methods.
 fluorescent protein into living cells and subsequently visualize the location and dynamics of the gene product using fluorescence microscopy.
fluorescent protein into living cells and subsequently visualize the location and dynamics of the gene product using fluorescence microscopy. The Strep-tag II is an eight-amino-acid peptide (WRHPQFGG), which, after fusion with the recombinant protein of interest, offers a practically useful solution to purify target protein. The Strep-tag II has negligible effect on the recombinant protein due to its chemically balanced amino acid composition. Generally, it does not interfere with folding or bioactivity and does not induce protein aggregation. Thus, there is no need for removing the tag.
The Strep-tag II is an eight-amino-acid peptide (WRHPQFGG), which, after fusion with the recombinant protein of interest, offers a practically useful solution to purify target protein. The Strep-tag II has negligible effect on the recombinant protein due to its chemically balanced amino acid composition. Generally, it does not interfere with folding or bioactivity and does not induce protein aggregation. Thus, there is no need for removing the tag. expression of the protein products transfected with cDNA constructs. The recognized KT3 epitope represents the amino acid sequence KPPTPPPEPET derived from the Simian Virus 40 (SV40) large T-antigen.
expression of the protein products transfected with cDNA constructs. The recognized KT3 epitope represents the amino acid sequence KPPTPPPEPET derived from the Simian Virus 40 (SV40) large T-antigen. Streptavidin is a 60kDa protein from Streptomycetes avidinii. The protein is a tetramer having four biotin-binding sites. Unlike avidin, streptvidin has a low isoelectric point (pI=5) and no carbohydrate groups, resulting in low nonspecific binding.
Streptavidin is a 60kDa protein from Streptomycetes avidinii. The protein is a tetramer having four biotin-binding sites. Unlike avidin, streptvidin has a low isoelectric point (pI=5) and no carbohydrate groups, resulting in low nonspecific binding. sometimes preferred to other fluorophores due to its colour, as well as its photostability compared to other monomeric fluorophores.
sometimes preferred to other fluorophores due to its colour, as well as its photostability compared to other monomeric fluorophores. Streptavidin is a 60kDa protein from Streptomycetes avidinii. The protein is a tetramer having four biotin-binding sites. Unlike avidin, streptvidin has a low isoelectric point (pI=5) and no carbohydrate groups, resulting in low nonspecific binding.
Streptavidin is a 60kDa protein from Streptomycetes avidinii. The protein is a tetramer having four biotin-binding sites. Unlike avidin, streptvidin has a low isoelectric point (pI=5) and no carbohydrate groups, resulting in low nonspecific binding. purified form, the protein of interest (X) is often cleaved from MBP with a specific protease. Protein X can then be separated from MBP by affinity chromatography.The fusion of proteins with MBP usually enhances their solubility and facilitates their proper folding so that the fusion proteins are most often bifunctional. In addition, such fusions can facilitate the crystallisation of difficult proteins, e.g. membrane proteins.
purified form, the protein of interest (X) is often cleaved from MBP with a specific protease. Protein X can then be separated from MBP by affinity chromatography.The fusion of proteins with MBP usually enhances their solubility and facilitates their proper folding so that the fusion proteins are most often bifunctional. In addition, such fusions can facilitate the crystallisation of difficult proteins, e.g. membrane proteins. Dextrin Resins is a chromatography medium for purifying proteins fused to maltose binding protein (MBP-tagged protein). Recombinant proteins with MBP-tags often gives increased expression levels and higher solubility of the target protein. Proper folding of the attached protein has also been shown to be promoted by the MBP tag. Since MBP increase the solubility, the tag is extremely useful for recombinant proteins accumulated in an insoluble form (inclusion bodies).
Dextrin Resins is a chromatography medium for purifying proteins fused to maltose binding protein (MBP-tagged protein). Recombinant proteins with MBP-tags often gives increased expression levels and higher solubility of the target protein. Proper folding of the attached protein has also been shown to be promoted by the MBP tag. Since MBP increase the solubility, the tag is extremely useful for recombinant proteins accumulated in an insoluble form (inclusion bodies). peptide sequence of the myc-tag is (in 1- and 3-letter codes, respectively): N-EQKLISEEDL-C, N-Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu-C, where N stands for Amino-terminus and C stands for Carboxy terminus. It can be fused to the C-terminus and the N-terminus of a protein. The tag is approximately 1202 Daltons in atomic mass and has 10 amino acids.
peptide sequence of the myc-tag is (in 1- and 3-letter codes, respectively): N-EQKLISEEDL-C, N-Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu-C, where N stands for Amino-terminus and C stands for Carboxy terminus. It can be fused to the C-terminus and the N-terminus of a protein. The tag is approximately 1202 Daltons in atomic mass and has 10 amino acids.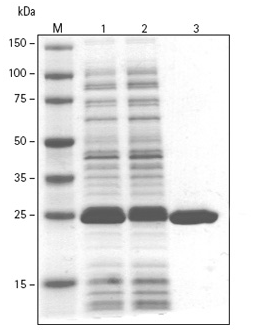 Glutathione S-transferase (GST) is a 26 kDa protein derived from Schistosoma japonicum. GST enzymes from various sources, both native and recombinantly expressed as fusion to the N-terminus of target proteins. Reduced glutathione (GSH), when immobilized as a ligand to agarose or other chromatography supports, enables high-yield and high-quality purification of recombinant proteins expressed as fusions with glutathione S-transferase (GST) or other glutathione binding proteins expressed in E. coli, insect cells and mammalian cells.
Glutathione S-transferase (GST) is a 26 kDa protein derived from Schistosoma japonicum. GST enzymes from various sources, both native and recombinantly expressed as fusion to the N-terminus of target proteins. Reduced glutathione (GSH), when immobilized as a ligand to agarose or other chromatography supports, enables high-yield and high-quality purification of recombinant proteins expressed as fusions with glutathione S-transferase (GST) or other glutathione binding proteins expressed in E. coli, insect cells and mammalian cells. prokaryotic expression systems.
prokaryotic expression systems. Glutathione S-transferase (GST) is a 26 kDa protein derived from Schistosoma japonicum. GST enzymes from various sources, both native and recombinantly expressed as fusion to the N-terminus of target proteins, are easily purified using glutathione (GSH) agarose beads is well documented and provides a one-step, high purity affinity purification. Due to the positive influence of the GST-tag on protein solubility and expression efficiency especially of small proteins, this technique has been widely used to generate proteins for crystallization, molecular immunology studies and studies involving protein-protein and protein-DNA interactions.
Glutathione S-transferase (GST) is a 26 kDa protein derived from Schistosoma japonicum. GST enzymes from various sources, both native and recombinantly expressed as fusion to the N-terminus of target proteins, are easily purified using glutathione (GSH) agarose beads is well documented and provides a one-step, high purity affinity purification. Due to the positive influence of the GST-tag on protein solubility and expression efficiency especially of small proteins, this technique has been widely used to generate proteins for crystallization, molecular immunology studies and studies involving protein-protein and protein-DNA interactions. transfer. The HA tag antibody can specifically recognize the C terminal or N terminal with HA tag (HA-tagged) fusion protein, and also can be used to detect the HA expression of tag fusion protein expression, cellular localization, and purification, qualitative and quantitative detection of HA expression of tag fusion protein.
transfer. The HA tag antibody can specifically recognize the C terminal or N terminal with HA tag (HA-tagged) fusion protein, and also can be used to detect the HA expression of tag fusion protein expression, cellular localization, and purification, qualitative and quantitative detection of HA expression of tag fusion protein.